Decades of Experience. Proven Results.
Bispecific
Monoclonal Antibody Development and Manufacturing
Talk to our Experts
KBI Biopharma: Offering a Leading Technology Platform Backed by Exceptional Scientific Experience
Bispecific monoclonal antibodies (bsAbs) are revolutionizing the biopharma industry, offering dual-target capabilities for enhanced therapeutic potential. By simultaneously engaging two distinct targets, bsAbs promise improved efficacy and broader applications, positioning them at the forefront of the next frontier in innovative drug development.
At KBI Biopharma, we've mastered the art and science of bsAbs. Following our operational consolidation with Selexis SA, we possess a combined proven track record of innovative solutions supercharged by the KBI Biopharma SUREtechnology Platform™, powered by Selexis®.
A recent study published in Biotechnology and Bioprocess Engineering highlights KBI Biopharma's technological approach to establishing cell lines that offer robust expression of bispecific antibodies at high yields (and with a high percentage of heterodimers).
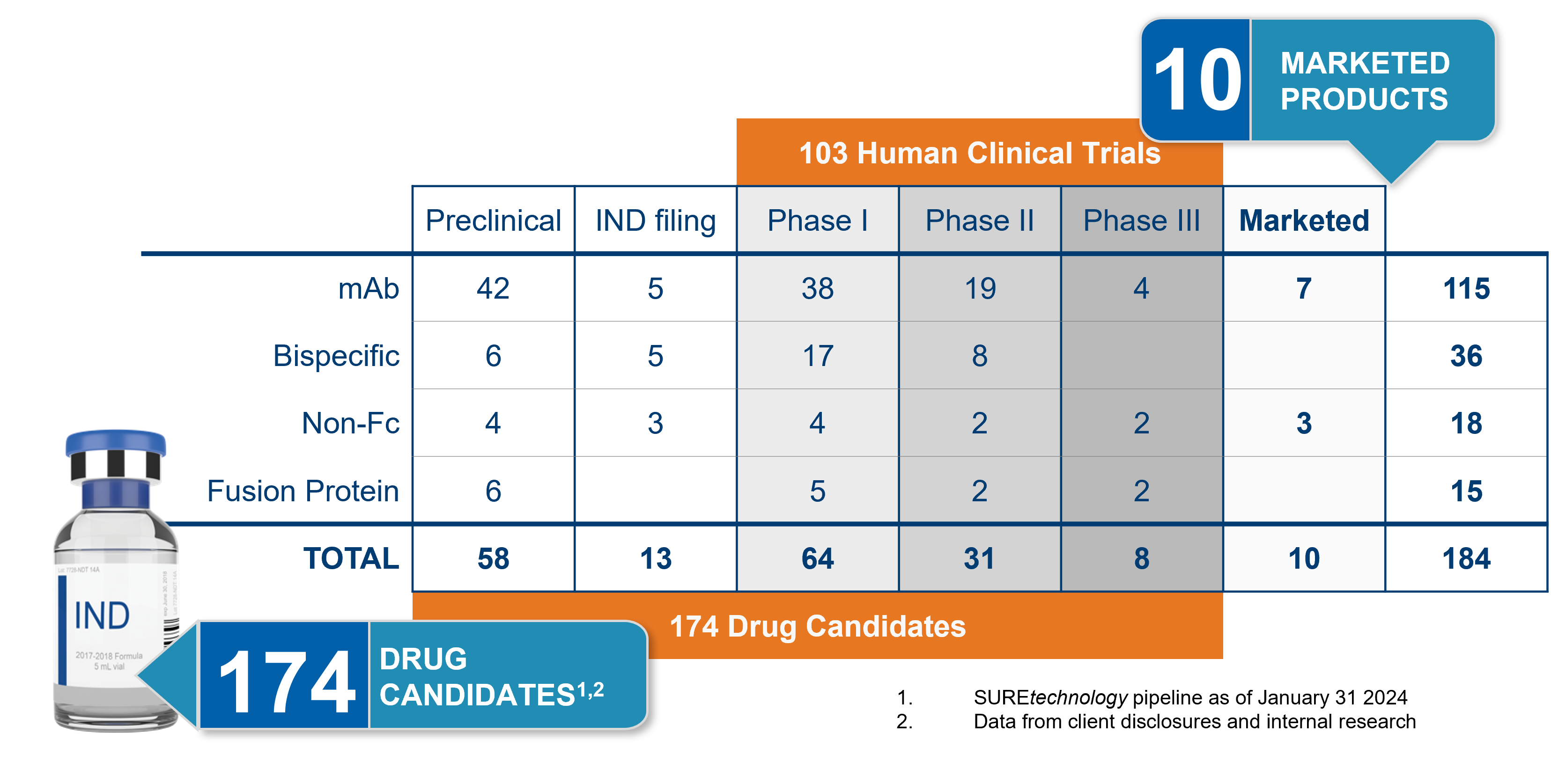
Our SUREtechnology Platform has successfully produced 36 bispecific antibody programs, 25 of which are currently in Human Clinical Trial stages.
Integrated Solutions for Bispecific Antibodies
Our fully-integrated development platform encompasses a strong understanding and efficient production of bispecific antibodies from cell line development (CLD) through GMP commercial manufacturing.
Why KBI Biopharma?
Proven Track Record
KBI Biopharma has over 20 years of experience in development, manufacturing, purification, analytics, characterization, and formulation of bispecific monoclonal antibodies.
Innovative Solutions
The SUREtechnology Platform is designed to overcome common expression bottlenecks in cell line development. We overcome the challenges of transcription, translation, DNA repair, protein folding, glycosylation, and secretion to bring you a premium suite of cell line development tools and technologies.
We also have expertise with bispecific monoclonal antibody process development, manufacturing, characterization and analytical method development and validation, and formulation.
Real-World Results
We have real-world examples of successful cell line generation and robust production of bispecific antibodies for clinical applications, including 6 bsAbs in preclinical stages, 5 IND filings, 17 in Phase I, and 8 in Phase II, as of January 2024.
World-Class Expertise in Bispecifics
Our seamless approach optimizes your experience, delivering high-quality bulk drug substances under accelerated timelines.
Having delivered preclinical proof-of-concept for our TCR Bispecifics program IMA402 and having moved this program into GMP process development is a major achievement of Immatics. We have chosen Selexis and KBI because of their expertise in the development and manufacturing of bispecific antibodies. We are looking forward to working with them on our path towards IND for IMA402
I am pleased to report the excellent progress we are making in developing CMX-02. By targeting both TNFα and IL-23 simultaneously I believe that CMX-02 can deliver important benefits for treating patients with severe autoimmune diseases such as psoriatic arthritis and Crohn´s disease. I am also excited about [using the SUREtechnology Platform] which will allow us to produce and develop this novel immunotherapeutic more quickly.
We are delighted to partner with [the SUREtechnology Platform], and access its proprietary protein expression tools and technologies, IP and know-how. The partnership is key to advancing our CDX [bispecific antibody] programme into clinical trials and accelerating the timeline to deliver this innovative therapy to patients in need of a more benign and effective treatment for AML.
Combining drugs is at the heart of therapeutic approaches to cancer. Bispecific antibody combinations have potency and targeting advantages that provide the basis for improved anti-cancer therapies. With the best-in-class RCB generation capabilities of [the SUREtechnology Platform] on board, the existing knowledge and infrastructure for the manufacturing of therapeutic monoclonal antibodies is optimally leveraged. This collaboration represents an important step towards affordable combination therapies for cancer.
Save Time, Maximize Quality.
Best-in-class CLD combined with unmatched expertise in mammalian-based contract development and manufacturing.
Talk to our Team of Experts
Let's Chat!
Fill out the form to get in touch with our team of experts for more information!
A Comprehensive Approach to Bispecific Antibody Production
At KBI Biopharma, we don't just focus on one aspect of bispecific antibody production - we address challenges in cell line development, production, purification, and manufacturability. Our innovative and advanced SUREtechnology Platform is designed to express a diverse range of molecules with high titers.
Learn more about our capabilities and depth of experience:
Publication:
Application of platform process development approaches to the manufacturing of Mabcalcin™ bispecifics
In their article, the authors evaluate a high-yield manufacturing platform using Mabcalcin™ molecules (which consist of Anticalcin® proteins fused to an IgG), assessed to commercially-relevant scales1. This platform approach demonstrates a fast, optimized route to process confirmation that aligns with classical monoclonal antibody (mAb) approaches when it comes to timelines and overall cost.
Whitepaper:
Delivering on the Promise of Bispecifics
Given the complexity of bsAbs, a development platform for these needs three key features to be sufficiently robust: Stable and high expression of bsAbs, straightforward early screening, and a robust cell line that can handle stressors. Furthermore, advancing bsAbs into the clinic requires process development, analytical methods, and scale-up for cGMP manufacturing. Our leverageable, integrated workflow generates high-quality clinical bulk drug substances under accelerated timelines.
Article on Labiotech.eu:
Overcoming the Challenges of Bispecific Antibody Production
In a recent article published on Labiotech.eu, Séverine Fagète, Vice President of Mammalian Cell Line Development at KBI Biopharma, details the innovative and advanced approach she and her team take to overcome common obstacles and bottlenecks in bsAb development and production. KBI Biopharma's strategic approach has been applied to more than 25 bsAbs, yielding up to 99% heterodimers and paving the way for efficient production of bsAbs for clinical use.
Webinar:
Development of effective purification processes for bispecifics using affinity capture and mix-mode polishing chromatography
Get an inside look at how KBI Biopharma and JSR Life Sciences have developed processes for the purification of bsAbs using Amsphere™ A3 affinity chromatography followed by two polishing steps. Here we discuss the challenges of bispecifics purification and our approach to mixed-mode chromatography designed for bispecifics.
Publication:
Tuning the Assembly of Bispecific Antibodies by Playing on Differential Polypeptide Chain Molar Ratios
KBI Biopharma’s Mammalian Cell Line Development team recently published the results of their research in Biotechnology and Bioprocess Engineering. In their article the authors evaluate the impact of manipulating different molar ratios of three polypeptide chains, concluding that by adding gene copies at specific ratios, the expression of bsAbs can be influenced beyond the initial engineering stage.
Webinar:
Streamlined Development For Efficient Production Of Bispecific Molecules Using An Integrated Platform Process
This webinar demonstrates a breakthrough platform approach that encompasses the efficient production of bispecific molecules in an integrated, streamlined way, from CLD (cell line development) to cGMP manufacturing. This integrated process results in high-quality bulk drug substances under accelerated timelines.
Talk to our Experts
© 2026 KBI Biopharma