About Us

As the icon of next-generation CDMOs, KBI Biopharma is driving breakthroughs in biopharmaceutical development and manufacturing that positively impact the lives of patients worldwide.
Our Values
Ownership
.png?width=661&height=602&name=MicrosoftTeams-image%20(86).png)
Collaboration
.png?width=662&height=602&name=MicrosoftTeams-image%20(84).png)
Excellence
.png?width=662&height=602&name=MicrosoftTeams-image%20(88).png)
Innovation
.png?width=662&height=602&name=MicrosoftTeams-image%20(85).png)
Our People
Our team, distributed throughout the US and Europe, is committed to a high standard of quality and execution. Each and every KBI employee takes pride in meeting the needs of our customers which directly impacts patients in need.
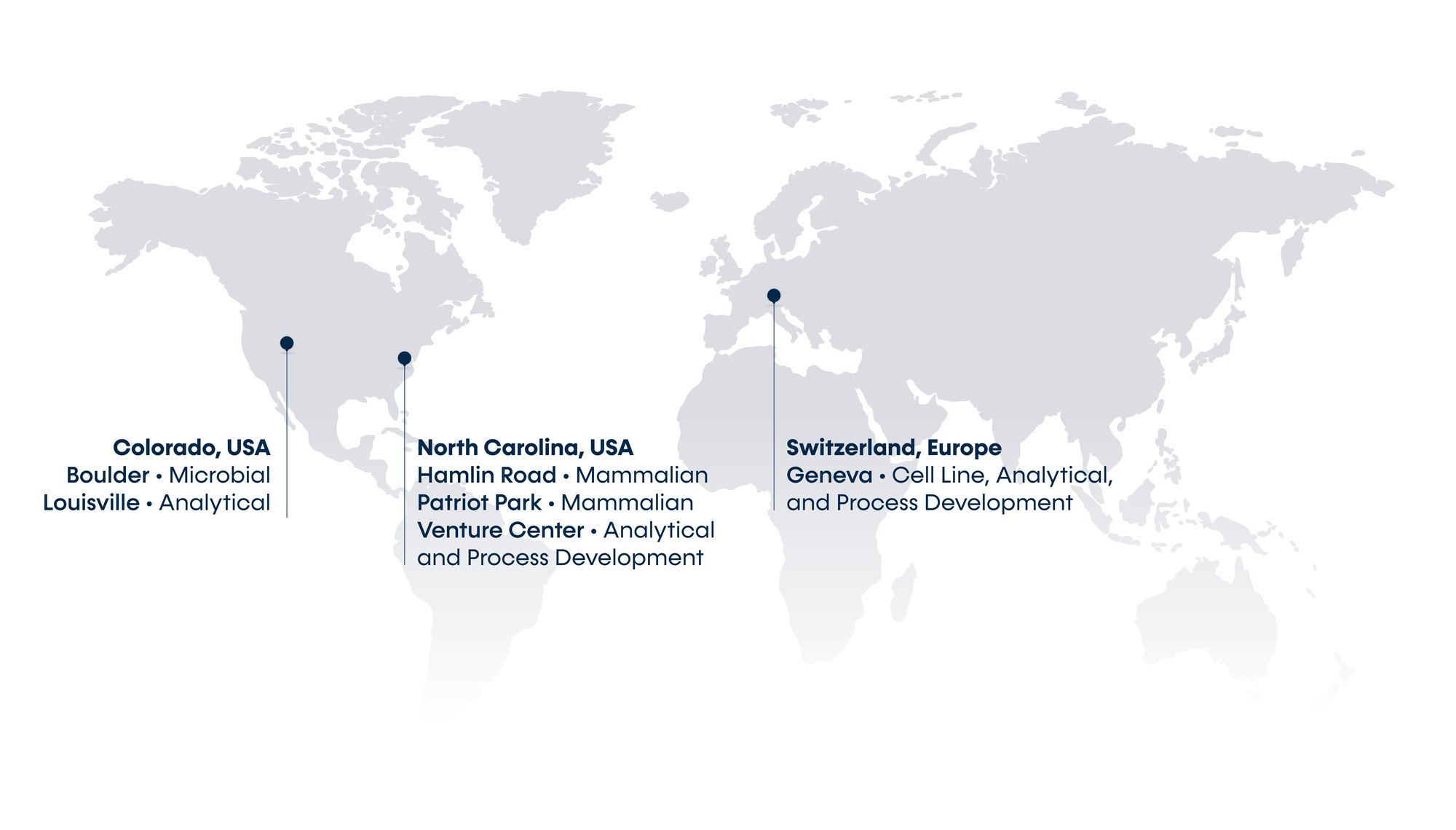
Our Locations
Built upon a foundation of world-class analytical capabilities, we deliver efficient process development and clinical and commercial cGMP manufacturing services for mammalian, microbial, and cell therapy programs. KBI has locations in Durham and Research Triangle Park (NC), Boulder and Louisville (CO), The Woodlands (TX), Geneva, Switzerland, and Leuven, Belgium.
Building a Sustainable Future
While KBI Biopharma serves a social purpose at its core, we recognize that there is also a need to take broader responsibility for the impacts of our business activities. To this end, we have aligned with our parent company, JSR Life Sciences’, Sustainability program in our focus and approach to Sustainability.
From an environmental standpoint, we’ve adopted a strategy of “Measure. Minimize. Mitigate.” and our approach is simple: No impactful change is too big to tackle or too small to dismiss. We want to accurately measure and drastically minimize our footprint through the adoption of energy-efficient technologies and processes, waste reduction, and conservation of natural resources and have set ambitious targets to do so.
Read our CEO's pledge to sustainability in full, here.
