Microbial
Raising the bar on microbial expression and manufacturability
Engineering New Biologics, At Scale
Optimization of Any Expression Strategy to Generate High Microbial-Based Expression for New Biologics
More molecules are manufactured using an E. Coli expression system than ever before thanks to reimagined platforms, efficiency gains, and broader-based applicability. KBI is raising the bar on microbial expression and manufacturing, using an early optimization workflow to ensure manufacturing success.
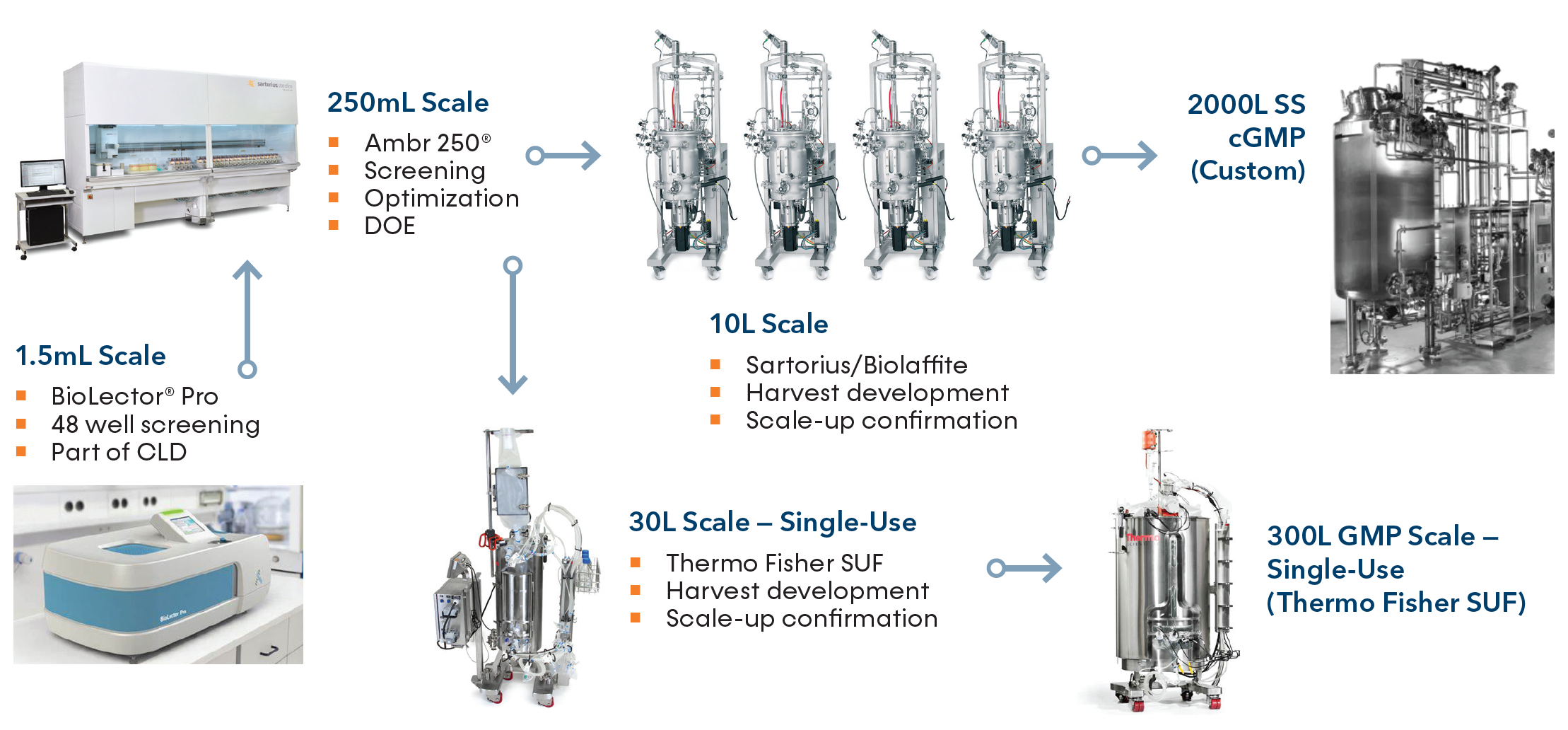
At KBI Biopharma, we are pushing the envelope on efficiencies with trusted microbial CDMO services. Our streamlined process scales from microfermentation to our largest working volumes, leveraging our CLD expertise paired with cutting-edge optimization to improve throughput and shorten timelines.
During this process, titers can be proportionally and accurately measured at very small volumes to identify a strain-plasmid combination that yields an optimized cell line. We quickly develop robust, scalable protein manufacturing processes through in silico protein analysis combined with wet lab IP and industry-leading screening workflows for biologics manufacturing using high-density microbial fermentation.
On average, KBI can generate a scalable process aimed at recombinant protein expression of >5 g/L in around 2 months. Our years of vertical process integration knowledge and data can allow us to streamline scalability.
Explore More Microbial:
Let's Chat.
Fill out the form below to get in touch with our team of experts.
See how KBI is Doing More with Microbes
The Right E. Coli Manufacturing Process
Starts with Early Screening and Optimization
Ahead of upstream process development, KBI focuses on microfermentation screening for microbial cell line development (CLD) to yield consistent, high titers across strains and molecules.
- Leveraging 10+ years of E. coli-based production and industry-leading microbial expertise
- Optimized CLD for secreted antibody fragments (FABs), inclusion bodies (IBs), and soluble intracellular molecules
- Heightened focus on screening scalable fermentation conditions
- Innovative workflows leveraging high-throughput robotics and microfermentation technologies
- State-of-the-art IP around our cell development and scalable processes
- Built for small- and large-scale cGMP manufacturing
- FDA/EMA Inspected Facilities
- Well-versed in first-in-human (FIH) studies, late-stage manufacturing, and commercialization
Microbial Cell Line Development
Biologics manufacturing by high-density microbial fermentation has advantages of development speed, low cost, and scalability.
- E. Coli expression systems
- Protein refolding with scalable processes for "difficult to refold" products
- High-density fermentation with rapid biomass production cycle times, scalable to 300 L to 2000 L scale
KBI applies proven, well-characterized microbial recombinant protein expression systems to provide state-of-the-art, early-stage/preclinical through GMP services.

Microbial Process Development
The clinical and commercial success of new biologics hinges on developing robust, reproducible, and scalable processes. Our process development activities cover the full development cycle:
- Early-stage discovery efforts
- Small-scale protein production
- Fully-integrated, comprehensive process development leading to GMP manufacturing
- Process characterization
- Scaled-down validation studies
Our deep knowledge and experience in the science and practice of biopharmaceutical drug development make us an ideal partner for process development.
Microbial Analytical Services
KBI employs a phase-specific lifecycle approach to analytics. Our experience includes antibodies like IgG1, IgG4, IgM, FAb, ADC, and Fc fusion, enzymes, cytokines, growth factors, highly glycosylated proteins, protein vaccines, PEGylated proteins, conjugates, peptides, adeno-associated viruses (AAVs), oligonucleotides, and other unique proteins.
- 3000+ analytical projects
- 100+ clients
- 130+ distinct molecules
Our expertise includes HPLC, CE, ELISA, UV-Vis, mass spectrometry, light scattering, biophysical characterization (DSC, CD, FTIR, fluorescence), binding assays (ELISA, Biacore, ForteBio), glycan analyses, cell-based assays, and others.
Formulation Development
Our approach to formulation development is based on the strategic pairing of two complementary scientific disciplines: First, establishing a comprehensive understanding of the protein's thermal, physical, chemical, and conformational stability, and second, employing statistical design-of-experiment (DOE) to evaluate the main effects and interactions effects on protein stability. Together, these techniques enable KBI to develop robust formulations by eliminating uncontrolled stability variables, thus focusing solely on therapeutic performance and clinical outcomes.
- 130+ successful protein, peptide, and vaccine formulation development programs
- Creation of robust formulations by eliminating uncontrolled stability variables
- Stable liquid formulations for protein concentrations ranging from >1mg/mL to 200mg/mL.
KBI's data-driven approach can also strengthen responses to regulatory inquiries.
Clinical Microbial Manufacturing
KBI has extensive experience in therapeutic protein and enzyme development, manufacturing, and commercialization. Our key personnel have led CMC projects through successful IND and BLA filings.
Our flexible application of stage-appropriate activities results in cost savings for early stages while providing the full regulatory support necessary to set up further advancement of programs into late-stage and commercial manufacturing.
Learn More: Clinical Manufacturing
Commercial Microbial Manufacturing
Our microbial facility is our center of excellence for microbial process development and GMP manufacturing. Using quality-by-design (QbD) principles to develop, qualify, and manufacture protein-based therapeutic processes, we have commercialized many products over recent years.
Introducing KBI's PUREplatform™
For Clean, Efficient Therapeutic Protein Production
Pushing the Boundaries of E. Coli Expression
Within a New Standard of Premium Microbial Cell Line Development
KBI has redefined what it means to have an optimal expression strain.
With 10+ years of therapeutic protein development rolled into one platform, the KBI PUREplatform starts in cell line development (CLD) with a proprietary strain platform that includes unique substrains, designed specifically to quickly feed upstream process development and late-stage manufacturing with preestablished process elements.
- Increased efficiencies
- Exceptional quality
- Engineered for commercial use
PUREcoli™
PURE in. PURE out.
Learn more about how to jump-start your microbial program with PUREcoli™ to greatly reduce impurities and reach higher titers.
Let's Chat.
Fill out the form below to get in touch with our team of experts.
Explore More Microbial Services & Capabilities:

Never Miss Another Update!
Subscribe to KBI's Newsletter, The Pulse, to stay up-to-date on all the latest news, articles, and events from KBI Biopharma.

.png?width=175&height=175&name=MicrosoftTeams-image%20(75).png)