KBI Biopharma's
SUREtechnology Platform™,
powered by Selexis®, introduces
SUREmAb™ CLD
Cell Line Development for Monoclonal Antibodies that takes you from transfection to Research Cell Bank in as little as 9 weeks*
Guaranteed minimum titers of 4 g/L at RCB stage, with additional guarantees after CLD*
Talk to our Experts
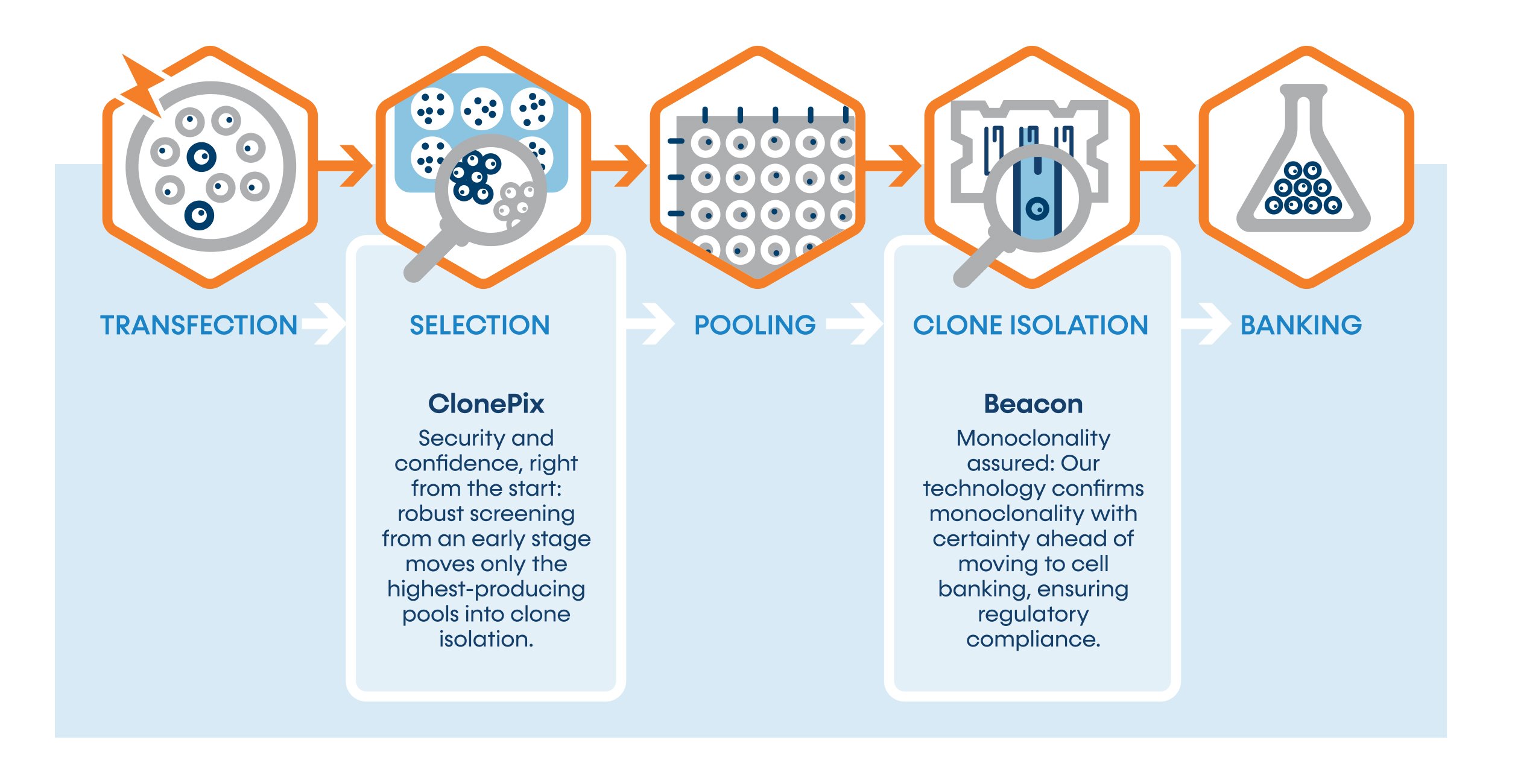
Streamlined Cell Line Development (CLD) Optimized for Monoclonal Antibodies
SUREmAb accelerates Monoclonal Antibody (mAb) CLD, bringing your project from transfection to Research Cell Bank (RCB) in as little as 9 weeks* with titers up to 18 g/L thanks to our high-performing CLD platform.
Built on the SUREtechnology Platform™
-
Leverages our proprietary, suspension-adapted SURE CHO-M Cell Line™ for fast doubling times of 15.6 hours and stability proven over 60 generations
-
Features transposase-mediated DNA integration for best cell candidates generation
-
Uses epigenetic elements that enhance transcription, contained in proprietary vectors optimized for the cell line
-
Harnesses efficient, scalable proprietary protocols and procedures specifically optimized for the SURE CHO-M Cell Line

Specific
Precision-engineered for a broad range of molecules

Unique
Proprietary platform built on patented technologies

Robust
Rapid, reliable, and versatile for producing recombinant proteins

Efficient
Streamlined and timely from early-stage to GMP manufacturing
Join a legacy of success with our SUREtechnology Platform™
0
Years of mAb Development Experience
0
Therapeutic mAb Projects
0
Commercialized mAb Therapeutics
Our Decades of Experience Have Enabled Us to Design an Optimal CLD Workflow for Secure, Speedy Development for Your Program
Your mAb CLD, De-risked for Acceleration
Clone Diversity
Throughout the development of your cell line, we ensure the clones we generate are diverse in genotype and phenotype to increase your program's resilience
Clone Invalidation
Our development strategies enhance DNA profile consistency from parental to daughter cells through the development of your monoclonal cell line, securing reproducibility of your testing
Clone Stability
De-risking stability studies are tackled ahead of Research Cell Bank delivery
RCB Guaranteed 4 g/L Titer*
Our intentional and optimized workflows ensures that we are developing only the highest-producing clones from an early stage
Enabling Your Program With Thoughtful Scale-up From the Start
Intentional Workflow for Rapid, Scaled Impact

Top-quality materials and methods for best-in-class development programs:
-
ClonePix™ enables a first screening of high producer pools from an early stage. By combining cell colony imaging technologies with labeling of your molecule, only the highest-expressing and viable pools pass the screening step. The robustness of the ClonePix step provides your project with a high degree of security and confidence, right from the start.
-
Our experts will select and load the most promising pools into Beacon® chips for export and clone isolation. This cutting-edge technology provides visual proof of monoclonality, ensuring the cell line is clonal in origin before moving to RCB banking. This method of monoclonality proof is commonly accepted as the highest proof by regulatory agencies, offering an even more robust confirmation of monoclonality than the traditional statistical calculation from FACS or limited dilution methodologies.
-
We make SURE your mAb will be expressed in the best cell line — one that is made to last and will drive you to the highest-possible production levels with minimal risk. Our RCB lead identification and stability assessment steps are both performed in 14-day fed-batch processes, in the ambr® 15 system. This system is a mini-bioreactor that is fully representative of the performance you can expect in scale-up to large volume manufacturing conditions. Additionally, the ambr® 15 system enables continuous and non-invasive monitoring and optimization of all conditions — including shape, materials, shear stress, stirring conditions, and fine-tuned monitoring of vitamins, nutrients, O2, pH, and other critical conditions that impact performance.
-
Within our SUREmAb offering, we have proven the stability of our proprietary SURE CHO-M Cell Line over 60 generations, ensuring the ongoing robustness and success of your CLD for your mAb project.
We Believe in Your Success
At the end of your CLD program with KBI Biopharma, you'll have monoclonal, high performing RCBs with a minimum guaranteed 4 g/L titer*, and a full data package that is ready for Investigational New Drug (IND) submission. We also offer a SUREmAb program that includes manufacturing, taking your project from transfection to tox material delivery in 5 months from a pool of clones and 6 months from top clones; or from transfection to cGMP Drug Substance release in as little as 11 months*. Upon continuation of manufacturing with KBI Biopharma, we offer a royalty-free option with alleviated license fees.
Development Success is a SURE Thing with SUREmAb
With optimized processes for efficiency and speed, SUREmAb delivers high titers with lower-cost workflows
Ask us about our royalty-free option
Innovation with Alleviation of Royalties
By continuing your project into manufacturing with KBI, we can waive the license fees for GMP clinical and commercial production - enabling you to make the most of our innovation without ongoing financial constraints. Fill out the form to get in touch with our team of experts for more information!
Global Compliance, Local Presence
KBI Biopharma is a trusted global CDMO with a strong track record in world-class cell line development, biomanufacturing, and analytics.

*Please note: The SUREmAb offer does not apply to mAb-based biosimilar projects or for any IgG shape-derived proteins that are different from an intact IgG format - including bsAbs, Fc fusions, and IgG fragments - as well as other protein classes outside of IgGs. Subject to terms and conditions.
Monoclonal Antibody Development, the Way it's Meant to be
KBI Biopharma is a trusted global CDMO with a strong track record offering best-in-class mammalian-based expression for breakthrough molecule types.
Talk to our Experts
© 2026 KBI Biopharma