Download the Poster:
Comprehensive Size Distribution Analysis of Adeno-Associated Virus Fill States
Comprehensive Size Distribution Analysis of Adeno-Associated Virus Fill States
Download the Poster
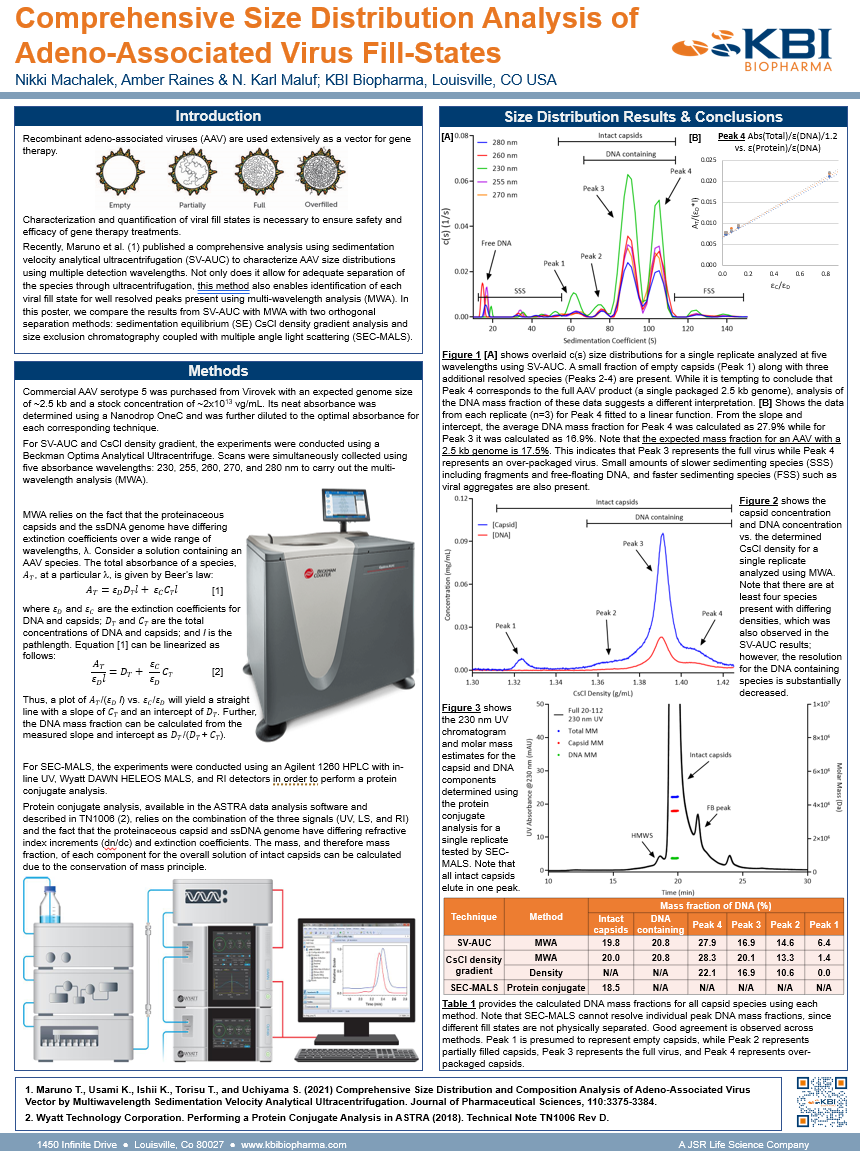
In support of developing “gold standard” analytical workflows that streamlines and optimizes characterization of the various capsid fill states that are present in AAV products, the team at KBI Biopharma performed a comprehensive and targeted comparison of SV-AUC combined with MWA with two orthogonal separation methods: sedimentation equilibrium (SE) CsCl density gradient analysis and size exclusion chromatography coupled with multiple angle light scattering (SEC-MALS).
The Right Data With The Right Insight
Analytics is a decision driver across every phase of a project cycle. At KBI, our deep expertise in analytics covers every stage of a project, from structure prediction and analysis to cell line development to commercial manufacturing - across non-cGMP and cGMP activities.
Questions? Comments? Talk to our Experts:
© 2026 KBI Biopharma